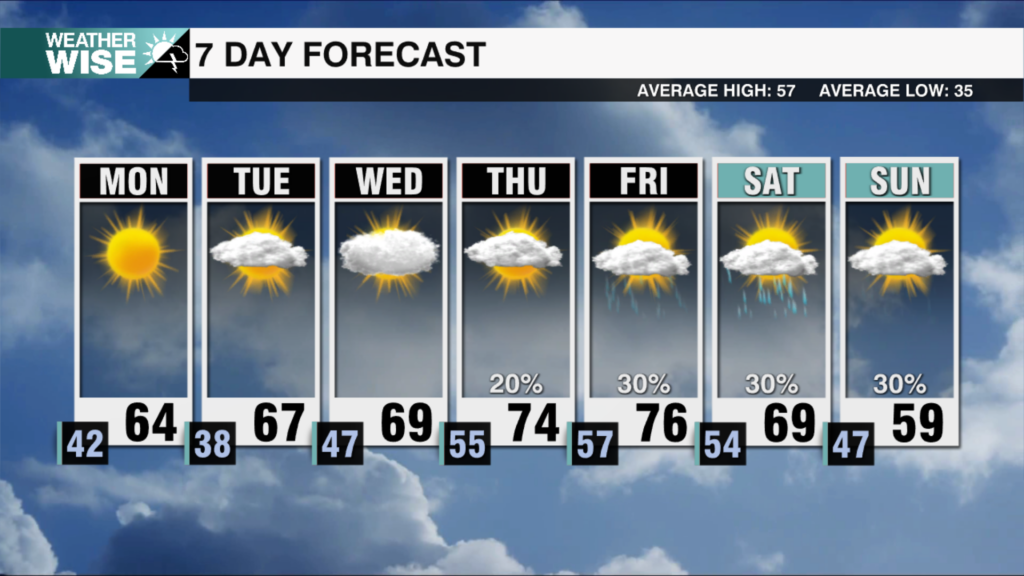
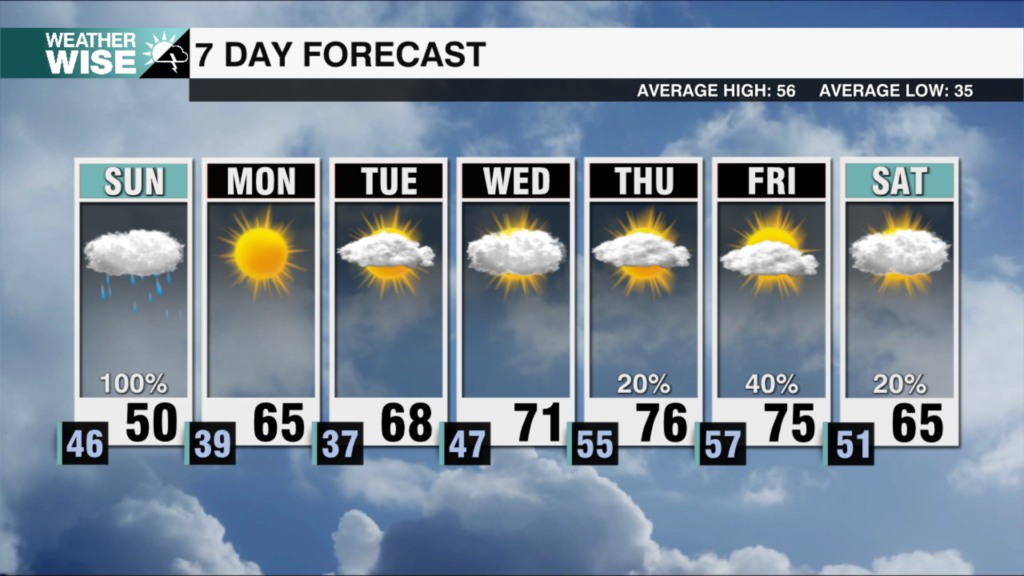
WASHINGTON — U.S. regulators are extending COVID-19 boosters to Americans who got the Moderna or Johnson & Johnson vaccine.
They also said Wednesday anyone eligible for an extra dose can get a brand different from the one they received initially.
The Food and Drug Administration announcement marks a big step toward expanding the U.S. booster campaign, which began with extra doses of the Pfizer vaccine last month. But it’s not the last word.
The Centers for Disease Control and Prevention will consult an expert panel later this week before finalizing official recommendations for boosters.